Hardy Diagnostics Test Detects COVID-19 in 15 Minutes
Santa Maria Company Releases New Rapid Coronavirus Test
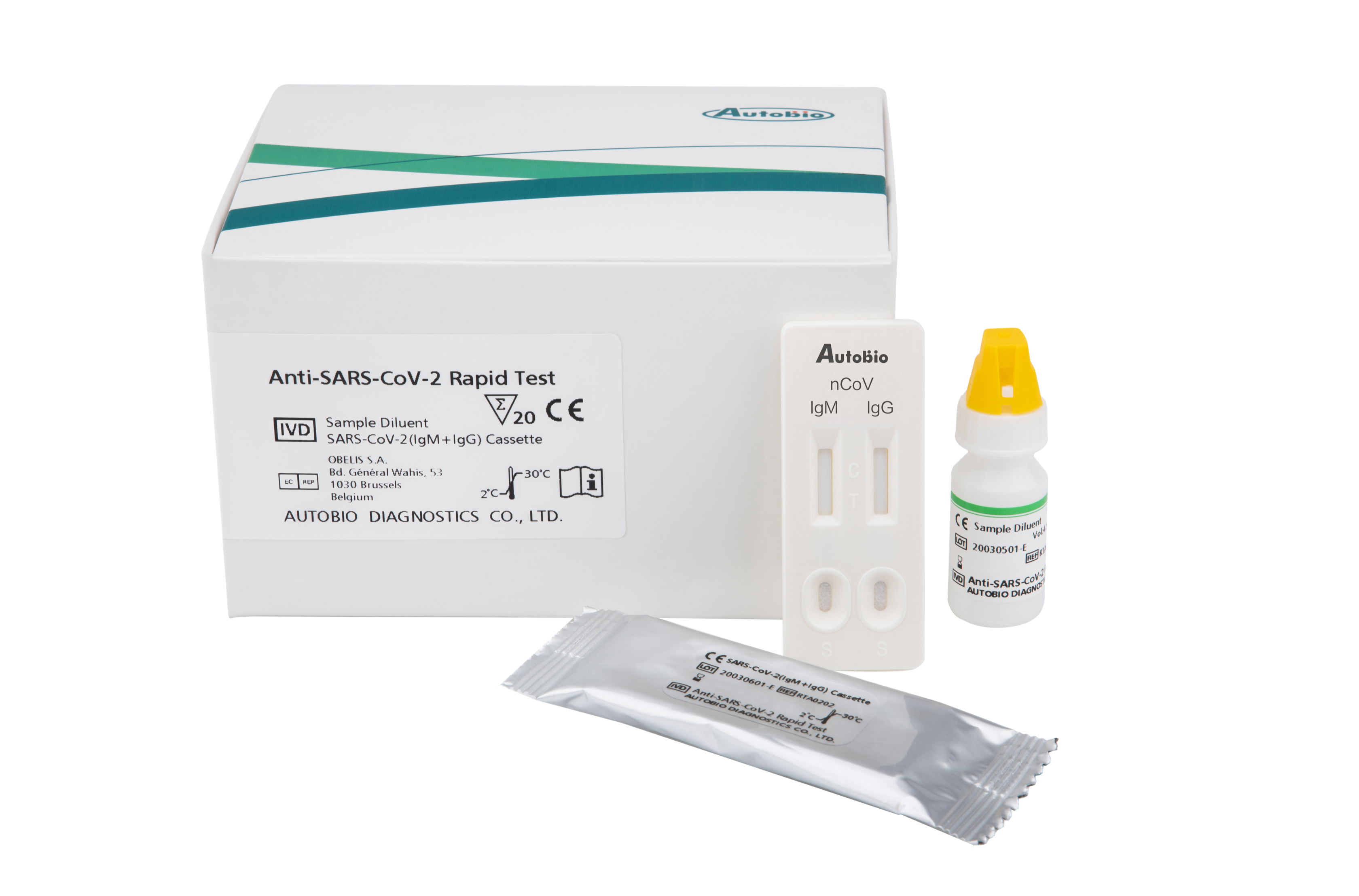
In just the past two days, Hardy Diagnostics in Santa Maria took more than 200 orders for its new COVID-19 rapid test since rolling it out this week, said Joel Tefft, marketing manager for the company. The test, developed by Autobio Diagnostics, Hardy’s partner in China, can identify in about 15 minutes the antibodies produced to combat the COVID-19 virus by using blood, serum, or plasma from a patient.
Though demand is high, the rapid test is still en route from China, said Tefft. They expect 3,000 kits to arrive in one or two weeks, each containing 50 tests. The Anti-SARS-CoV-2 Rapid Test is not a home test kit — it can only be performed on a doctor’s order by a laboratory, Tefft advised — but it allows doctors to quickly begin appropriate treatment and quarantine protocols for the wildly contagious disease.

Autobio Diagnostics had worked to develop the test since January, when the novel coronavirus now causing a worldwide pandemic was first diagnosed in Wuhan. Headquartered in Zhengzhou, the capital city of the central Chinese province of Henan, Autobio had worked with Hardy before, said Tefft, and approached the Santa Maria company to speed the test through the Federal Drug Administration process, where the test is still pending approval.
Like many tests and therapies produced for the ongoing pandemic, Hardy’s rapid test falls under the Emergency Use Authorization of the Federal Drug Administration. It can detect the antibodies — soldiers of the body’s immune system that form about a week after infection — made to fend off viruses similar to SARS-CoV-2, which causes COVID-19, and a molecular diagnostic test is needed for definitive confirmation.
As well as the rapid test, Hardy Diagnostics makes and ships hand sanitizer, transport media for test samples, and facemasks — almost entirely with components from the United States — for which demand is currently outstripping production. A million surgical masks are on order from its manufacturing partners, said Tefft, as are millions of dollars worth of a solution from Italy to preserve the virus from test samples. Until the latter arrive, Hardy’s workhorse viral transport medium is revving up but without swabs, which carry precise requisites for efficacy and avoiding cross-contamination. They, too, are nearly unobtainable, and Hardy is pursuing them through its U.S. suppliers.
The company’s founders, biologists Jay Hardy and Rob Shibata, got their start in the lab at Cottage Hospital back in 1979, though Shibata joined his family’s NorCal business in 1983. Hardy now occupies a 76,000-square-foot campus not far from the Santa Maria Airport. The bread and butter for the 40-year-old company, said Tefft, is its microbiology media manufacturing. It’s grown to nine locations around the country, all of which are as busy as its headquarters in Santa Maria.
“The community is a ghost town,” said Tefft, “which is strange but good. It means everyone’s staying inside.” Even with operations running at full tilt, they practice social distancing and hand sanitizing and use electronic media to communicate. “One hundred percent of our employees are embedded in this,” Tefft explained. “They take personal pride in what they’re doing. They know it’s worth it.”


You must be logged in to post a comment.