Showtime for Stem Cells
Talk About Potential
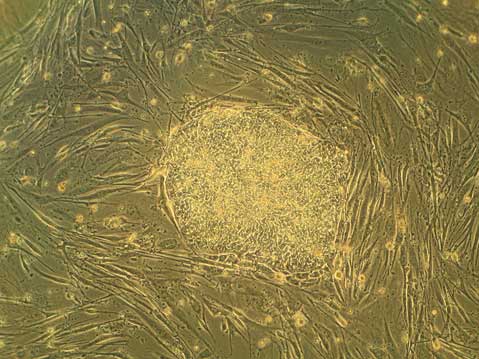
Just last month, the California Institute for Regenerative Medicine (CIRM) announced it was awarding over $250 million to multidisciplinary research teams to develop stem cell-based therapies to treat human diseases.
Of this money, CIRM is awarding $20 million for a multi-institutional grant on using human embryonic stem cells for vision research, with a focus on macular degeneration. Researchers at the University of California, Santa Barbara, are part of these efforts and will receive $2.5 million. Using human embryonic stem cells in vision-restoring treatments is just one of their many potential applications.
Last year, human embryonic stem cells (hESCs) celebrated the 10th anniversary of their discovery, and in the decade since their isolation they have received a great deal of press coverage about their many potential applications, as well as debate over ethical concerns. The decade indeed saw much progress in better understanding these cells and their capabilities.
The hESCs hold much potential for use in the field of regenerative medicine, which includes restoring damaged body tissues, organs, and limbs. They also hold great promise for serving as cellular models of human development and diseases, to give us a better picture of these promises. However, due to ethical and application concerns, only in January of this year were these cells approved by the F.D.A. for use in clinical trials.
Embryonic stem cells from other animals were successfully isolated long before human ESCs were. Nearly 30 years ago, in 1981, two research groups, one headed by Dr. Gail Martin at the University of California, San Francisco, and one a collaboration between Dr. Matt Kaufman and Dr. Martin Evans at the University of Cambridge in England, independently reported the isolation of mouse embryonic stem cells (mESCs). The mESCs were isolated from early-stage mouse embryos, approximately four to six days post-fertilization (out of 21 days total for mouse gestation). It was not until 1995 that this feat was accomplished with non-human primates by Dr. James Thomson’s group. And then, only a few years later, in 1998, embryonic stem cells were isolated from humans, once again by a group led by Dr. Thomson, who holds a faculty appointment at the University of Wisconsin and at UCSB.
It is important to understand where hESCs come from in order to understand the ethical arguments that surround them, as well as their enormous, innate biological potential. After the human egg is fertilized by the sperm, the resultant single cell (called a zygote) starts dividing and multiplying. To get hESCs, cells are isolated from early-stage embryos four or five days post-fertilization (they have not yet implanted in the uterus). At this point, the fertilized egg has developed into a hollow, fluid-filled sphere made up of approximately 150 cells. This stage is called a blastocyst.
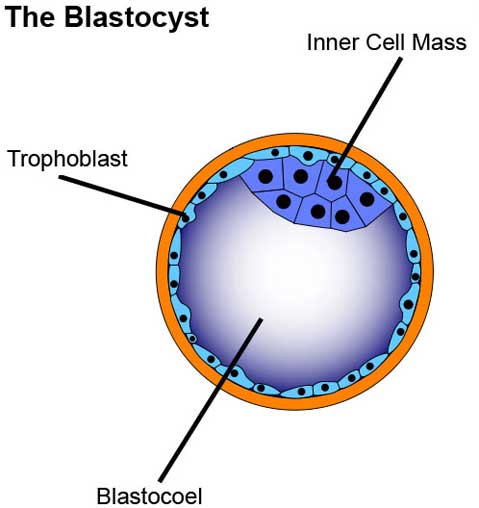
The blastocyst contains three distinct areas: the trophoblast, which is the surrounding outer layer of cells that later becomes the placenta; the blastocoel, which is a fluid-filled cavity within the blastocyst; and the inner cell mass, which is a cluster of cells inside the blastocyst that can become a fetus.
Embryonic stem cells can be created from cells taken from the inner cell mass. Because these cells are taken from such an early stage in development, they have the ability to become cells of any tissue type, which is called being “pluripotent.”
The pluripotency of hESCs is probably the trait that contributes most to their enormous potential, as cellular models of human development and disease, as well as for use in regenerative medicine. This trait is what mainly separates hESCs from adult stem cells, which are called multipotent or unipotent, meaning they are only able to become a more select group of cell types. Adult stem cells are also more limited in their cellular lifespan compared to hESCs.
Because these cells are taken from human embryos, researchers have taken many careful steps to address ethical concerns. For the original creation of hESCs in 1998, blastocysts used were donated with full donor consent from in vitro fertilization (IVF) clinics. Additionally, many researchers use blastocysts that would have been discarded by the IVF clinic because the embryos were damaged in some way and would never develop properly.
Researchers have even found ways to isolate hESCs from embryos while leaving the donor embryo intact and potentially able to develop normally; even earlier in development than the blastocyst stage, when the developing embryo contains only eight to ten cells, researchers have shown that they can remove one of these cells and create a line of hESCs and the remaining cells will continue to function as usual. The National Academies has also developed extensive guidelines of ethical standards for researchers to follow.
Though many obstacles have delayed hESCs from reaching the clinic, much progress has been made in making these cells suitable for clinical use. Because of their pluripotency, one defining feature of hESCs is the regrettable tendency to create a tumor when injected into a mouse. These tumors, called teratomas, are made up of a wide variety of different cell types. To prevent the formation of tumors, all hESCs used in therapies must first be completely turned into the desired target cell type; this process of making a stem cell become a mature, more limited cell type is called differentiation. Researchers have developed methods to completely differentiate hESCs to desired cell types, as well as many methods to confirm that they do indeed have a population that only contains these differentiated cells. Completely differentiated hESCs should not form teratomas and may be safe for therapeutic uses.
Additionally, hESCs are often grown with other supportive cells, called “feeder” cells (as is shown in the top image). Many such feeder cells are of mouse origin. This raises concerns of non-human contaminants in hESC cultures, although it is an area of much study, and many alternative methods that can create completely animal-free culture systems have been made.
Lastly, there is challenge of making patient-specific cells from hESCs, which is less of a problem when using many adult stem cells. However, this last problem, along with aforementioned ethical concerns, is being addressed with the creation of hESC-like cells from adult cells, termed induced pluripotent stem (iPS) cells. Human iPS cells were created in 2007 independently by two groups, one headed by Dr. James Thomson and the other by Dr. Shinya Yamanaka at Kyoto University, Japan.
Overall, hESCs have made much progress in the decade since their discovery, despite hurdles set before them. In March of this year, many previous political restrictions on hESC research were removed by President Obama. Just the month after that, the Geron Corporation received Food and Drug Administration approval for the first clinical studies using hESCs, specifically to treat patients with acute spinal cord injury. These first clinical studies hopefully mark just the beginning for more clinical studies using these very promising stem cells.
For more on human embryonic stem cells, see Teisha Rowland’s “All Things Stem Cell post on Human Embryonic Stem Cells,” The National Institute of Health’s Stem Cell FAQs, or, for a visual explanation of terms used, see All Things Stem Cell’s Visual Stem Cell Glossary.
Biology Bytes author Teisha Rowland is a science writer, blogger at All Things Stem Cell, and graduate student in molecular, cellular, and developmental biology at UCSB, where she studies stem cells. Send any ideas for future columns to her at science@independent.com.



