Hardy Diagnostic’s Rapid COVID-19 Test on Hold
Santa Maria Company’s Chinese Partner Caught Up in Blanket Prohibition
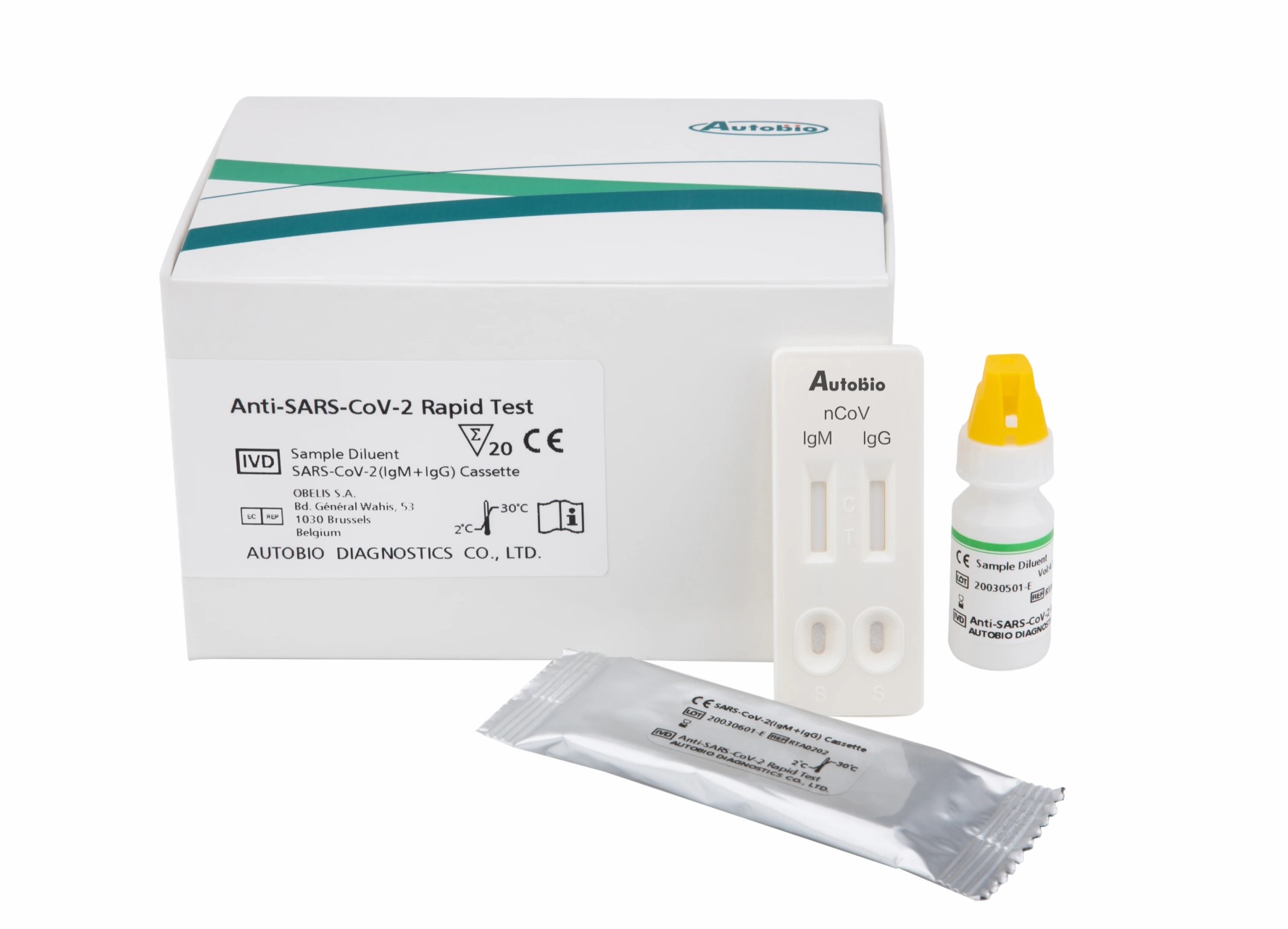
In the world of things going wrong, the latest victim is Autobio Diagnostics’ rapid COVID-19 test coming out of Santa Maria’s Hardy Diagnostics. According to Hardy Diagnostics’ president, Jay Hardy, another company’s test was halted mid-shipment by the Chinese version of the FDA, and Autobio Diagnostics — Hardy’s partner in China — got caught up in the resulting blanket prohibition. Hardy’s company had been ready to ship hundreds of orders of the 15-minute test last week.
Hardy said the Autobio test had been 100 percent proven at Marian Regional Medical Center in Santa Maria, as well as in validation tests in China and Europe, where it is currently in use. Marian conducted 10 tests of positive and negative samples using Autobio’s rapid kit, said laboratory chief Dr. Kevin Ferguson, and all were correct.
Marian, which is part of the large Dignity Health hospital chain, is awaiting a shipment of the 45-minute test from Cepheid today, Ferguson added, a small company in Sunnyvale. It identifies genetic material from the SARS-CoV-2 virus, which is the gold standard to determine COVID-19 infection. Shipments went to sister hospitals last week, Ferguson said, and Marian’s been allocated 180 this week. Whether they’ll get them all is another story, he said, as the quantities are determined by areas most in need. The Dignity group, which includes CommonSpirit Health, has hospitals in New York, which is experiencing a crushing surge of cases and deaths.
The bad test kits blocked by the Chinese government, said Hardy, had only a 40 percent success rate at picking up the virus. The test Hardy is importing identifies antibodies to the virus and can also detect immunity to the virus. The way it works is that the test has two wells for blood samples. One well can detect iGm antibodies, or the body’s quick response to an ongoing infection. The second divot detects iGg antibodies, or the longer-term antibodies the body later produces when the infection has ended. In that second sense, it can tell if a person has antibodies and possible immunity to COVID-19. Hardy was not aware of any studies that had yet visited reinfection.
Hardy’s rapid test got pre-approval from the Federal Drug Administration’s Emergency Use Authorization (EUA) program, which allowed the import and sale of the Autobio test. The FDA looked at the previous studies done in the European Union and China before issuing the EUA, said Hardy, including its approval in the EU.
“There’s no final ruling,” Hardy said, “but they seem okay with what we provided to them.”
The Cepheid test is also under EUA approval, an emergency measure given the need to test for the COVID-19 virus.
Dr. Ferguson, who oversees the labs at the French and Arroyo Grande hospitals, too, took a minute to explain why wearing masks and staying six feet apart mattered right now. “We know there are large numbers of asymptomatic people [with the virus], but we can’t do large-scale testing,” he said, as test-making production in the U.S. is still ramping up. “And we don’t want to strip the ability to take care of each and every patient [by overwhelming the hospitals]. That’s why the social distancing measures put in place are so important.”
At the Santa Barbara Independent, our staff continues to cover every aspect of the COVID-19 pandemic. Support the important work we do by making a