In vitro Fertilization, Part I: A Brief History
Accounts for More than One Percent of U.S. Births
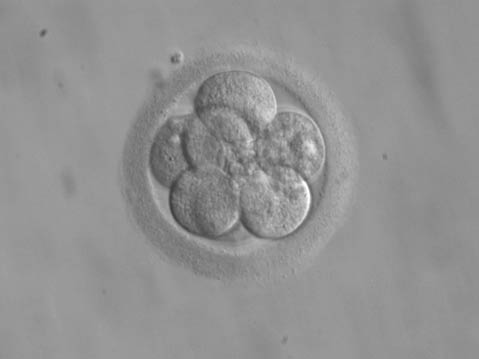
Infertility is an extremely prominent issue for many couples. One solution that increasing numbers of people turn to each year is in vitro fertilization (IVF).
Infertility Infertility is a very real, very silent, and very chilling health issue that many couples are forced to confront. One in ten people in the United States will face fertility problems. For a woman, these issues are closely linked to her age: When a woman is in her late 20s, her fertility starts to decline. It falls even more quickly when she turns 30, and continues to decrease by 3 to 5 percent each following year. It falls even more quickly after she’s 35, and plunges when she becomes 40. Combined with male fertility problems, by the age of 30, seven percent of couples are infertile, and this increases to 33 percent by the age of 40.

These statistics combined with the strong desire to have one’s own biological child drive about one million people in the U.S. to seek fertility treatment of some kind every year, and some 300,000 resort to IVF. For many, it works. Each year, over 1 percent of the babies born in the U.S. are through IVF. That’s around 50,000 births. The industry of assisted reproductive technology (ART) is booming. Fertility drugs bring in about $3 billion annually, and there are over 500 fertility clinics in this country alone.
But how well does IVF actually work? The most successful fertility clinics report over 30 percent pregnancy rates. However, this means that some 60 percent of the trials, at least, do not result in a pregnancy. That success ratio is disappointingly low to many couples, especially in light of the costs both fiscal and emotional. IVF is very expensive, sometimes costing over $10,000 for one treatment, making it simply out of reach for most lower- and middle-class couples. And, because of the 30 percent success rate, many couples have to go through repeated treatment cycles to become pregnant, and many give up. It can be a treadmill: the hopeful couple keeps trying, thinking that the next cycle will be the one that brings a child into their lives.
But for many it does work, giving them a biological child of their own which they could probably otherwise not have, using an approach that was probably not available to their parents.
How did IVF come to be? Although IVF has only recently been widely used to treat infertility problems, people have been seeking medical help to overcome reproductive barriers for a long time.
In 1884, the first known artificial insemination using donated sperm took place—and the woman inseminated did not even know what had happened. A couple having fertility problems were examined by Dr. William Pancoast of the Jefferson Medical College in Philadelphia. After finding that it was the husband’s sperm that was the problem, and the woman’s reproductive system was fine, the doctor realized their fertility problems could be solved using donated sperm through artificial insemination. This had not been done in humans previously, and such an idea at the time would have been rather scandalous, possibly even considered to be an act of adultery.
Consequently, the woman, and most likely the husband as well, were not told what was causing the fertility problems, or what the “treatment” would be. She was told only to return for a follow-up appointment to have “her” fertility problems fixed. When she arrived, she was knocked out with chloroform and the doctor chose a sperm donor from his medical students that were present. The student masturbated into a cup and the semen was injected into the unconscious woman’s uterus. It was successful: Nine months later she gave birth to a baby boy, still not knowing how she became pregnant.
Not until the 1930s did the idea of artificial insemination receive much publicity. During the 1940s, researchers found how to successfully freeze donated sperm, which led to many sperm banks opening in the 1970s. However, artificial insemination is only beneficial when the infertility of a couple is due to the “male factor.” If the woman is having reproductive problems, the situation can be much more difficult to solve, although this was greatly helped with the development of pregnancy-stimulating hormones in the 1960s. However, because such drugs can only help so much, the arrival of IVF revolutionized the existing field of assisted reproductive technology.
Like all science, IVF didn’t come out of nowhere; researchers had been performing the studies that enabled the development of IVF for around half a century. In England in 1890, Walter Heape transferred embryos from one rabbit to the reproductive tract of another rabbit, and successfully had the recipient rabbit give birth to offspring using this method. For decades, more animal studies built upon Heape’s original findings.
But scientific research cannot progress without the proper tools. Techniques for culturing cells improved during the 1960s and 1970s and, together with a better understanding of the hormones released during pregnancy, this allowed researchers to eventually culture embryos outside of the human body.
In 1978, the first person was born from IVF. Lesley and John Brown had been trying for nine years to have a child, but one of Lesley’s fallopian tubes was blocked and consequently her eggs could not travel down to the uterus to be fertilized. On their mission to have their own biological child, Mr. and Mrs. Brown met Drs. Robert Edwards and Patrick Steptoe, who had been working on IVF treatments. Previous couples had tried IVF, but none of the treatments resulted in successful pregnancies. However, the IVF treatment worked for the Browns and on July 25th, 1978, Louise Brown was born.
Many feared that a child born through IVF would be abnormal, or ostracized by society, but as Louise was shown to be a healthy, happy baby, and accepted by others, these fears mostly subsided. Louise Brown is still doing quite well; she has a naturally conceived child of her own and will turn 32 at the end of this month.
So what exactly is IVF? In essence, in IVF sperm and eggs are removed from donors and fertilized outside of the body (in a Petri dish) and then implanted back into a woman’s uterus. (The term “in vitro” literally means “within the glass” in Latin and is often applied to the study of a normal biological process replicated outside of an animal. In the case of IVF it is a bit misleading as the embryos are not placed in a glass tube, but in a plastic Petri dish.)
One treatment, or “cycle” of IVF, takes about four to six weeks from start to finish. Before the eggs can even be removed from the woman, her ovulation cycle is strictly regulated using drugs that first prevent ovulation (for two weeks) and then drugs that stimulate ovulation (for about ten days). This helps ensure that she will have a large number of eggs ready to be harvested at a given time. As her ovulation cycle is being regulated, her developing eggs are monitored through ultrasound, and her hormone levels are observed through blood samples. About 34 to 40 hours before the eggs are to be harvested, the woman is given a final drug to “ripen” the eggs. The woman is then anesthetized and a fine needle is guided to her ovaries and her eggs are removed. The eggs are mixed with the sperm from the husband, partner, or donor. If all goes well and the eggs become fertilized, the resultant embryos (fertilized eggs) start growing in a Petri dish in the clinic.
Only a few days after the eggs are removed, the best embryos are selected to be implanted; the embryos have about eight cells at this point. A thin catheter is used to insert the embryos, usually one or two, back into the uterus. (This is easier than removing the eggs; no anesthetic is used.) Two weeks after implantation, the woman undergoes a pregnancy test; if it is positive, the pregnancy should progress normally.
Intracytoplasmic Sperm Injection While IVF has helped many couples conceive their own biological children, the “male factor” was still often a problem. Many couples bypassed this by using donor sperm, but this meant that the “recipient father” would not be the child’s biological father. To help satisfy men’s desires to more easily be biological parents, a procedure called intracytoplasmic sperm injection (ICSI) was created in the early 1990s. Previously, in order to fertilize an egg in IVF, hundreds of thousands of sperm were required. However, for ICSI to work it theoretically needs only one functional sperm. In ICSI, a single sperm is taken and inserted directly into the egg, meaning that extremely low sperm counts are no longer necessarily a significant barrier to having children.
The ICSI technique has become so popular that in 2005 ICSI was used in 60 percent of the IVF treatments, even though only 37 percent of the IVF cases had a problem with the “male factor.”
ICSI has dramatically changed the fertility industry. The success of ICSI has had other consequences. It’s believed that heterosexual use of sperm banks is declining. However, the use of sperm banks by single women and lesbian couples is actually increasing.
Consequences of IVF Now that people have access to the amazing reproductive tool of IVF (and often in conjunction with ICSI), what are the consequences, socially, scientifically, and ethically? Biology Bytes will explore these topics in next week’s article, which will look at the legalities surrounding the practice of IVF, the isolation of embryonic stem cells, the creation of “designer babies,” the occurrence of multiple births (twins, triplets, and more!), concerns of a growing infertile population, and the growing practice of surrogacy.
For more on in vitro fertilization, see the book From IVF to Immortality by Ruth Deech and Anna Smajdor, the book Test Tube Families: Why the Fertility Market Needs Legal Regulation by Naomi R. Cahn, the book In Vitro Fertilisation in the 1990s by Elisabeth Hildt and Dietmar Mieth, the Center for Disease Control’s website on the 2007 Assisted Reproductive Technology Report, and Wikipedia’s article on “in vitro fertilization.”
Biology Bytes author Teisha Rowland is a science writer, blogger at All Things Stem Cell, and graduate student in molecular, cellular, and developmental biology at UCSB, where she studies stem cells. Send any ideas for future columns to her at science@independent.com.



